
Three-dimensional protein structure is directly correlated with its function and its determination is critical to understanding biological processes and addressing human health and life science problems in general. Laboratory of Genetics and Molecular Cardiology, Heart Institute, University of São Paulo Medical School, São Paulo, Brazil.Not all proteins have a quaternary structure, especially not structural proteins.Letícia M.
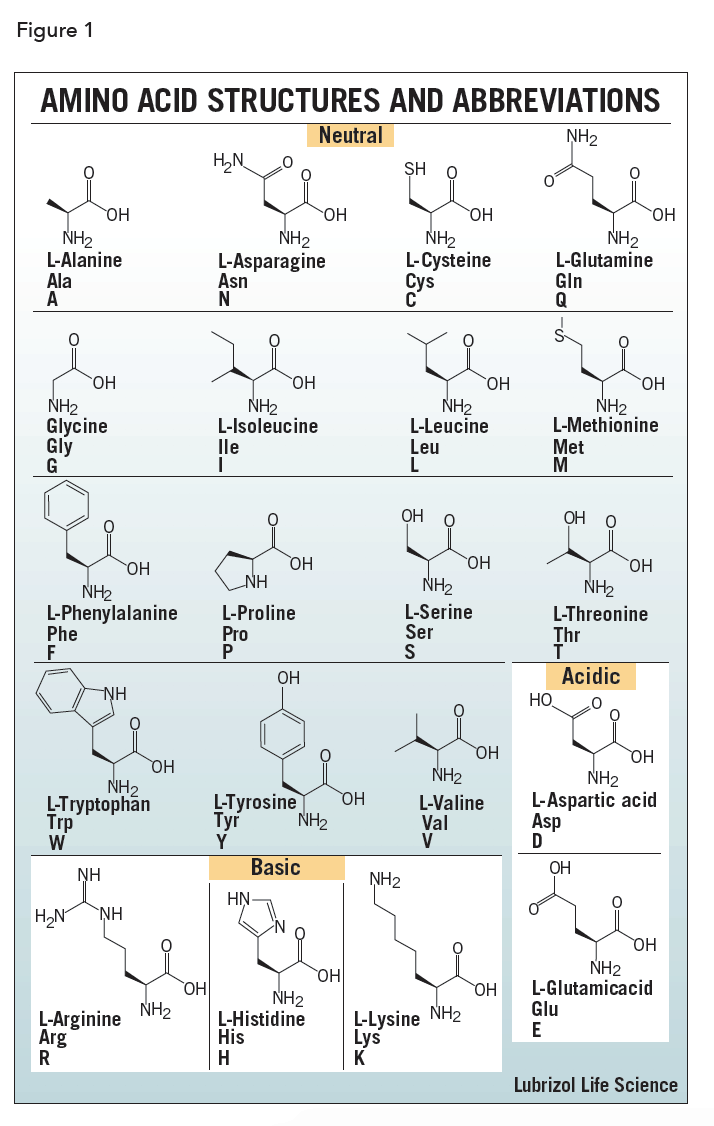
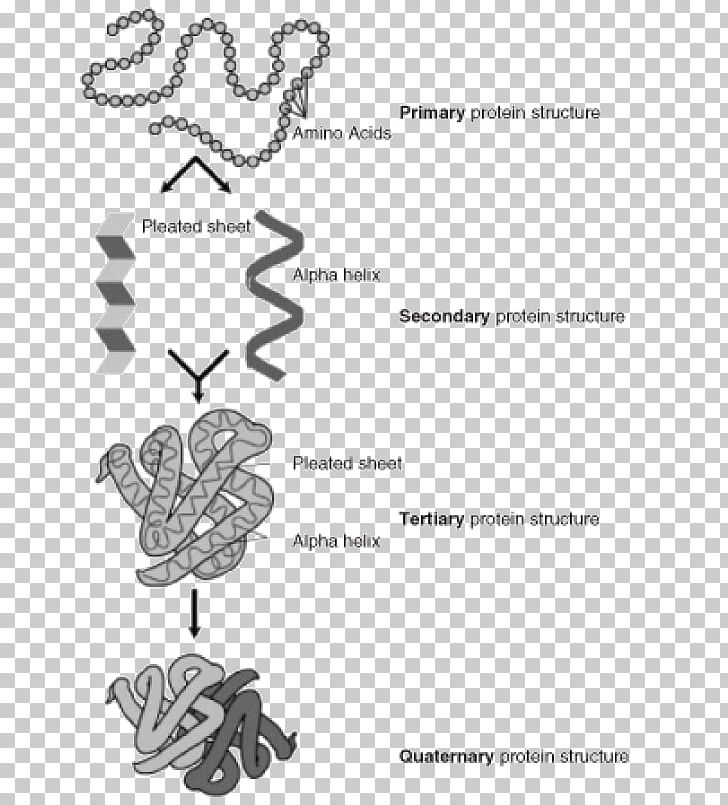
The polypeptides are bound non-covalently, and the prosthetic group assists the protein carry out its function better. In the case of haemoglobin, there are four polypeptides: two alpha chains, two beta chains and one prospethic group, haem, per polypeptide (so four in total on one molecule). Lastly, the quarternary structure is the combination of many tertiary structure proteins and sometimes, a prosthetic group. Finally, there are also hydrogen bonds between R-groups (not between the peptide groups but the R-groups - peptide group hydrogen bonds are used in secondary structure). There are also hydrophobic interactions, where hydrophobic residues prefer to be in the center of the protein, and the hydrophilic ones face outwards. The thiol groups are oxidised to form a sulphur-sulphur bond, and this helps maintain the 3D shape of the protein. Disulphide bonds/bridges are covalent bonds between the R groups of two cysteines, which have a -S-H (thiol) group on the end. Salt bridges/ionic bonds/charge-charge interactions form between positively charged (usually basic residues such as lysine) and negatively charged (such as glutamic acid) residues. This is where amino acid specificity comes in the R-groups of the residues (that is, the variable groups attached to the alpha-carbon of amino acids) allow for certain bonds to form. Tertiary structure is concerned with the 3D folding of the protein. Meanwhile, beta pleated sheets are a bit looser, and have inter-molecular hydrogen bonding, where many polypeptides can be bound together. This bonding stabilises the structure, and is intra-molecular hydrogen bonding (the same strand forms bonds to itself). A alpha helix is a tight coil (like a spring), with the peptide groups of residues forming hydrogen bonds with the ones above and below it. The secondary structure of a protein can either be an alpha helix or a beta pleated sheet. Each amino acid (also referred to as a residue when it is in a polypeptide) is attached by a covalent peptide bond one either side, and each amino acid has specific properties which will allow for the protein to take the correct shape. The primary structure of a protein is the newly translated polypeptide strange of amino acids, which are in the order as dictated by its mRNA. Protein folding needs to be specific, and repeated exactly the same way every time for the same protein. The structure of proteins, especially those of enzymes, is extremely important in allowing them to carry out their duties in the cell and body.


 0 kommentar(er)
0 kommentar(er)
